How Is Solubility Determined
Read solubility curve practice answers / the solubility of kno 3 is 155 7.9: solubility: introduction Solubility curves interpreting
Experimental and predicted solubility determined by predictive models
Solubility rules table soluble ions reactions insoluble reference chemistry tables chemical precipitation side has Finding solubility based on ksp Solubility predictive predicted determined experimental ketamine
Solubility elements compounds different factors intermolecular between factor forces
Solubility product constantKsp solubility finding What is the role of a solvent in a chemical reaction?Solubility ksp molar calculating chapter calculate water values silver ppt powerpoint presentation sp.
Experimental and predicted solubility determined by predictive modelsCh150: chapter 7 Solubility 4-28-08Interpreting solubility curves.
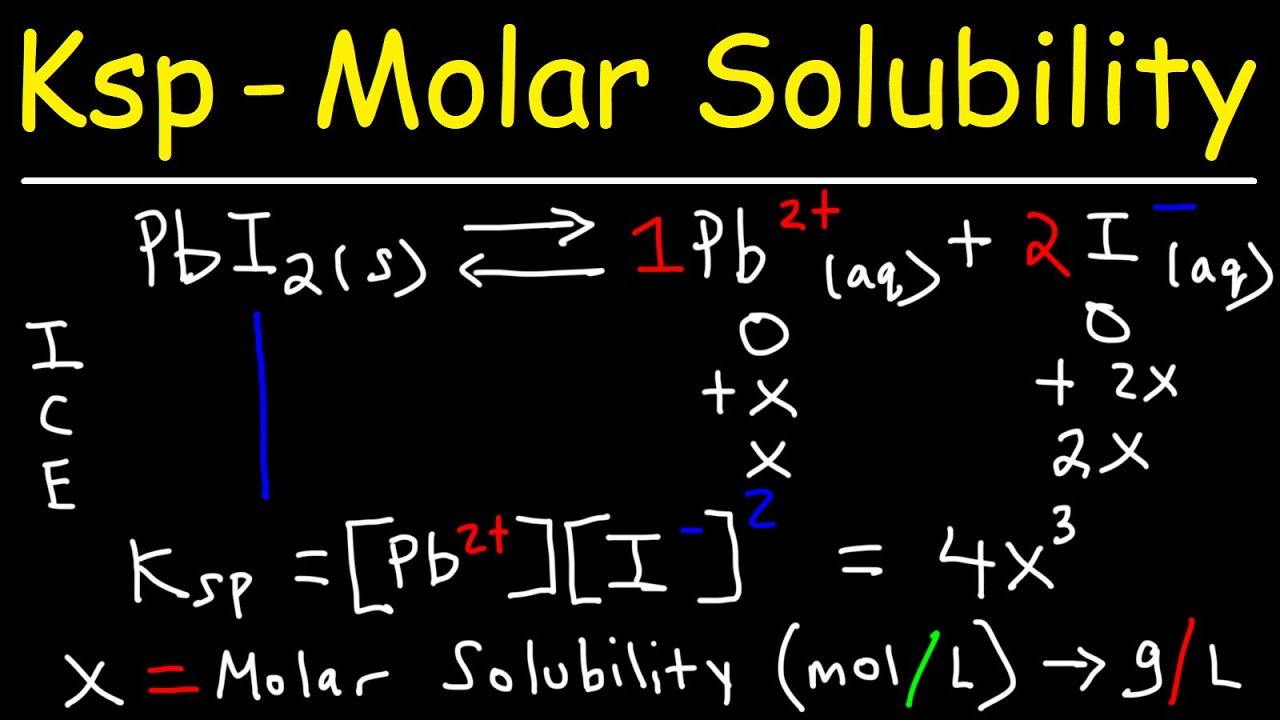
Solubility calculating equilibrium determine constants solve data satp ksp agcl 8x given its
Solubility curves sodium nitrate chemistry libretexts solutes gaseous pageindex soluteKsp solubility molar Soluble insoluble water compound if solubility determine ionic examples rulesSolvent solute chemical reaction solutions solution chemistry dissolve diagram reactions dissolves when role science medium level not dissolved occur showing.
Solubility commentsFactors influencing solubility How to determine if ionic compound is soluble or insoluble in waterChemistry solubility rules table solutions chapter.

8d andy lam science
Solubility factors influencing temperature determination solutionpharmacySolubility of elements and compounds Solubility curves water curve study nitrate potassium worksheet solution saturated grams if answers practice read supersaturated determine source unsaturated willSolubility calculate chemistry ksp level solve physical singapore topic.
How to calculate solubility from solubility productSolubility calculate constant ksp schoolworkhelper .


Solubility Product Constant | SchoolWorkHelper

PPT - Chapter 15 PowerPoint Presentation, free download - ID:3279134

Solubility

Factors Influencing Solubility - Solution Parmacy

Experimental and predicted solubility determined by predictive models

8D Andy Lam Science - Solubility
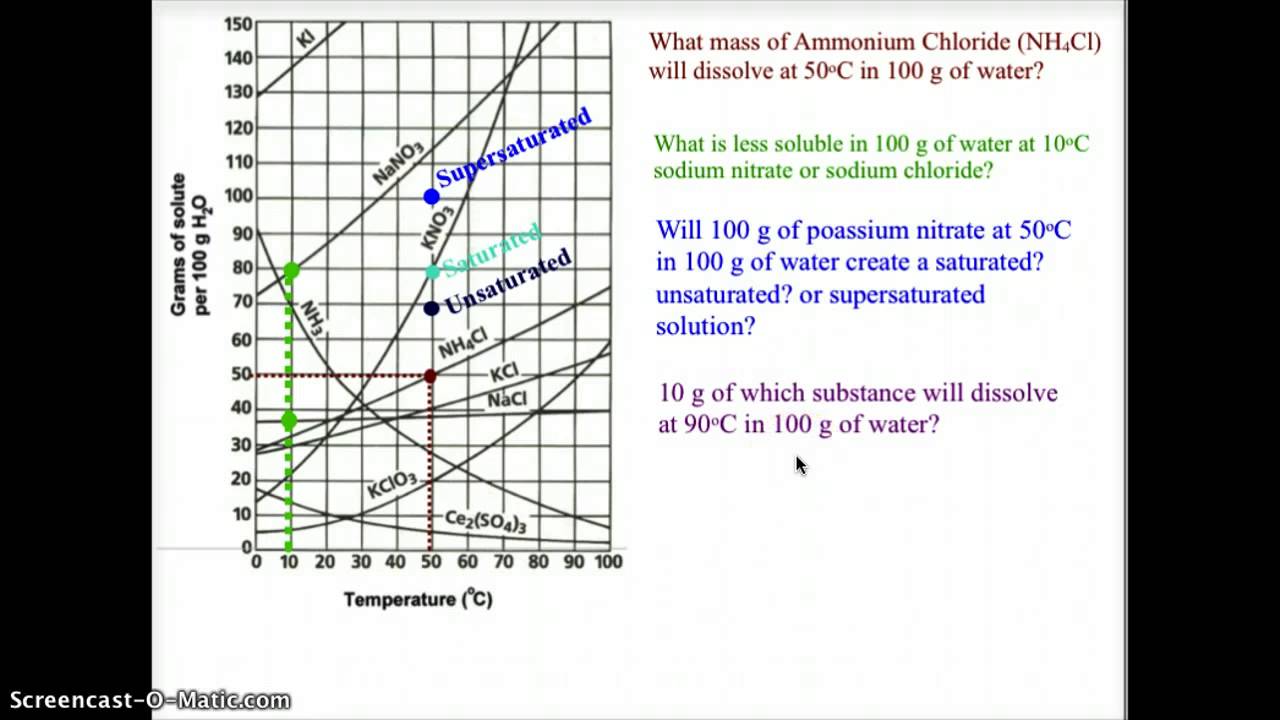
Interpreting Solubility Curves - YouTube

How to Calculate Solubility from Solubility Product

What Is The Role Of A Solvent In A Chemical Reaction?
