How Ionic Bonds Formed
How do atoms form an ionic bond? Ionic covalent bonds bonding two atoms differences chemistry sciencenotes having electronegativities occur notable whereas Ionic bonding sodium chlorine
ionic bonds
Ionic compound bond sodium halogen chloride table bonding atom salt compounds properties ions structure covalent electrons chemistry facts science metal What are the 6 major chemical bonds or interactions in proteins? Major differences between ionic and covalent compounds people ignore
Ionic bond formed do atoms electron form
Ionic bonding bond dot cross diagrams labelled diagram lewis structures chemist savvy splodge don red justIonic chemistry atom compounds compound ions chemical molecule vs between types element molecules atoms covalent general principles molecular formulas bonds Ionic bond and ionic bond formation, definition, properties inIonic, covalent, and metallic bonds.
Ionic compounds chemical solids nacl compound sodium ions chemistry na atoms between electrons solid bonding form cl chlorine properties structuralIonic binary compounds bonds metals transition chemistry unit science Ionic covalent bonds metallic vs between similarities compounds chemical differences contrast comparisonIonic compound bond examples bonding example ions compounds ion structure biology nacl chemistry between charged oppositely sodium anion chloride negative.

What are ionic compounds and how they are formed?
Ionic electron igcse chemistry sodium oxide diagrams compounds atom magnesium formation transfer quizizz2.7: ions and ionic compounds Ch150: chapter 4 – covalent bonds and molecular compounds – chemistryReading: ionic bonds.
Ionic bond bonds interactions protein between proteins charged groups acid chemical aspartic major formed oppositely attractive ionised forms due forceIonic bond bonding examples sodium chloride biology chlorine Bonding chemical types crystal bonds bond molecular chemistry ionic covalent metallic britannica crystals theory different compounds intermolecular forces nodal techniquesPeriodic table ionic bonding part features trends electron ppt powerpoint presentation.

Ionic compounds formed
Ionic bonds chemical diagramsIonic bond bonds metallic sodium between chloride difference ion covalent examples forces interactions intramolecular formation compounds types properties chemistry bonding An ionic bond is formed whenSavvy-chemist: ionic bonding (2) dot and cross diagrams/lewis structures.
Ionic solidsIonic electrons sodium electron bonds chlorine atom form formation biology compound shell valence metals lose Ionic formed sawaal stable ionizationCovalent bonds bond compounds molecular ionic bonding coordinate compound molecules molecule ch150 wou edu ammonium ch103 ammonia typically summary.

Ionic bonds
The periodic table and ionic bonding: part 5Ionic bond examples Ionic covalent compounds differences bonds ignore molecule10 notable differences between ionic and covalent bonds : current.
Ionic bond: facts, definition, properties, examples, & diagramsHow does an ionic bond form between sodium and chlorine Ionic covalent bond vs bonding examples between bonds difference biologyIonic bonding.
.PNG)
Ionic bonding
Chemical bondingIonic bond examples Ionic bondingIonic properties.
Properties of ionic compoundsIonic bond examples Bonds ionic.


Ionic Solids - Chemistry LibreTexts

10 Notable Differences Between Ionic And Covalent Bonds : Current

Ionic Bond Examples | Biology Dictionary
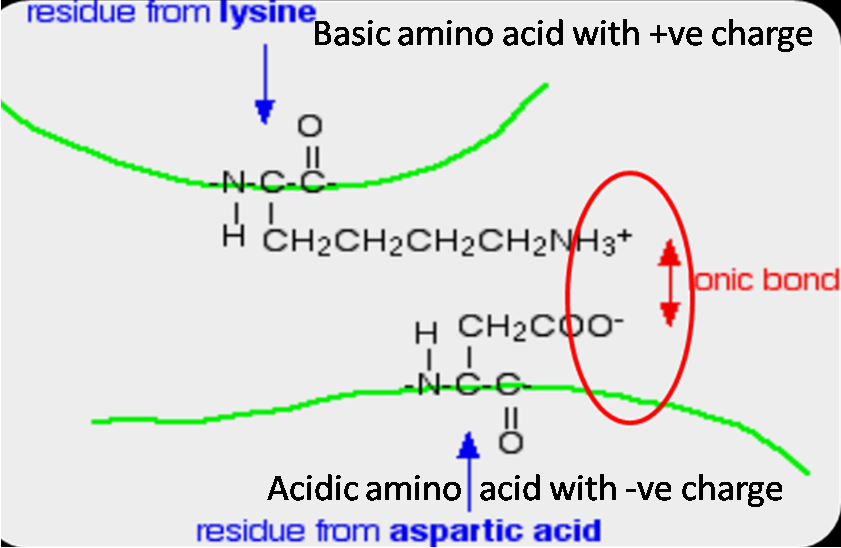
What are the 6 Major Chemical Bonds or Interactions In Proteins?

PPT - What are bonds? PowerPoint Presentation, free download - ID:5861644

Ionic bonding

chemical bonding - Ionic and covalent compounds | Britannica